- The Barn Holly Berry House Rough Park,
Hamstall Ridware, Rugeley, Staffordshire, WS15 3SQ - 666-888-0000
- info@elarapharma.com
- info@elarapharma.com
- The Barn Holly Berry House Rough Park,
Hamstall Ridware, Rugeley, Staffordshire, WS15 3SQ - Contact Us
Oct2018
Elara Pharmaceuticals Ltd helps Valiseek® demonstrate efficacy of its lung cancer therapy in phase 2 clinical trial
Elara Pharmaceuticals Ltd helps Valiseek® demonstrate efficacy of its lung cancer therapy in phase 2 clinical trial.
Tersan®’s KEM® data analysis shows positive results for ValiSeek VAL401 in Phase II trial for non-small cell lung cancer (NSCLC) patients.
Tersan’s KEM® (Knowledge Extraction and Management) advanced Artificial Intelligence technology, shows that patients receiving the VAL401 treatment have statistically significant improvement in Overall Survival compared to those receiving no treatment, as well as a measurable improvement on Quality of Life.
These results identify an overall response rate in the per protocol population of 60%.


“Palliative stage patients could expect to see improvements in symptoms with the added benefit of improved survival prospects. This encouraging 60% overall response rate seen in this first all-comer trial provides a strong foundation for the next stage of clinical testing” Dr Suzy Dilly, ValiSeek CEO.2


(ValiRx – Company Update – Tue, 06 Feb 2018)
“ The team at ValiSeek, Tersan and ValiRx have been instrumental in ensuring this data is presented and interpreted fully…” Dr Suzy Dilly, CEO of ValiSeek.3
I look forward to the“successful crystallisation of substantial value for patients and shareholders”, Dr Satu Vainikka, ValiRX CEO.1
“The company has seen considerable progress across our therapeutic portfolio in the last six months, adding value to the company’s assets and bringing us closer to the point where our clinical programs for VAL401 will deliver meaningful data and will bring the company closer to finding a partner. I am pleased with the momentum generated as we move towards clinical development a milestones and potential value inflection points for VAL401 as we approach the year-end,” Oliver de Giorgio-Miller, Non-Executive Chairman.4
This case study illustrates Tersan’s ability to create value for potential patients and its clients, managing the complexity of analyzing small datasets. KEM® is a comprehensive and FDA-tested clinical data analysis system that enables full exploitation of complex and small datasets

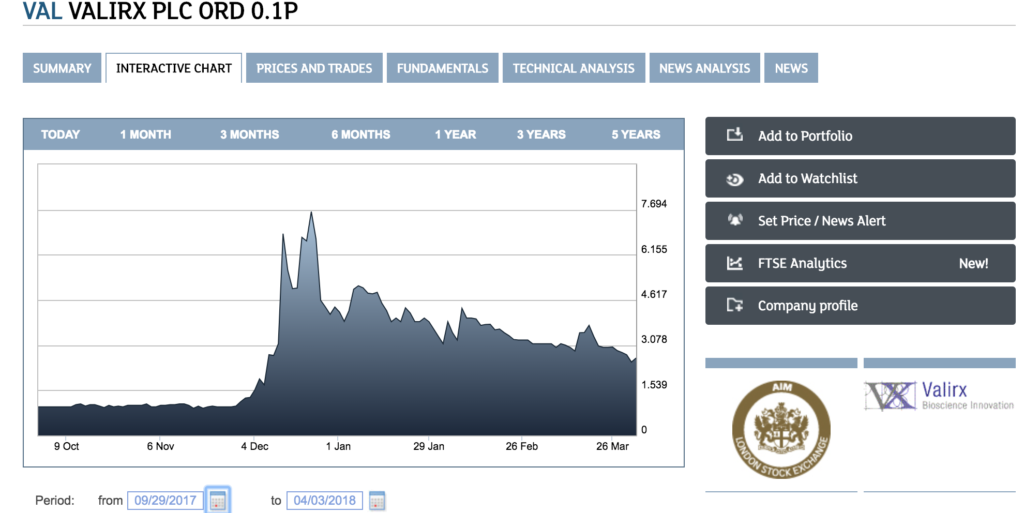
Source : https://www.londonstockexchange.com – VAL VALIRX stock value from 09/23/2017 to 04/03/2018
Further reading
6 Sep 2018 – ValiRx – Update on Clinical Development – [Press Release Document] (1)
14 Jun 2018 – ValiRx – VAL401 Update – [Press Release Document] and [Audio document]
10 April 2018: – ValiRx – Final Results for the year ended 31 December 2017 – [Press Release Document]
06 Feb 2018 – ValiRx – VAL401 Update – [Video]
16 Jan 2018 – ValiSeek – Clinical Development Update on VAL401 – [Audio Document] and [Press Release Document] (2)
16 Jan 2018 -VAL401: A Novel Cancer Therapeutic (Updated Jan 2018) – [One Pager Document]
12 Dec 2017 – ValiSeek – Clinical Development Update on VAL401 – [Audio Document] and [Press Release Document] (3)
12 Dec 2017 – ValiRx unveils positive results from VAL401 Phase II lung cancer trial – [Video]
26 SEP 2017 – ValRx – Half Year Report – [Press Release Document] (4)
-
Tags:
- cancer, recent post

